Explain How Synthesis Reaction Differs From a Decomposition Reaction
A B -- AB. We have to compare and contrast synthesis reaction and decomposition reactions.

Synthesis And Decomposition Reactions Youtube
A decomposition reaction produces multiple products from a single reactant.

. Synthesis reactions are one of the major classes of chemical reactions which. In a synthesis reaction bonds are formed to join two or more atoms or molecules to make a new compound. Two reactants are combining and form one product.
One way to think of synthesis reactions is that they are the reverse of a decomposition reaction. Describe the factors that can affect the rate of chemical reactions. Decomposition reaction is the opposite of synthesis decomposition reaction is where one single compound breaks down into two or more simpler substances.
Chemical decomposition is the effect of simplifying a complex chemical entity into a single chemical entity into two or more fragments. AB A B. Describe synthesis decomposition and exchange reactions giving an example of each.
Combustion reactions are the combination of some compound with oxygen to make oxides of the other elements as products although nitrogen atoms react to make N 2. A single substance reacts to make multiple substances. A composition reaction produces a single substance from multiple reactants.
A synthesis reaction occurs when two or more reactants combine to form a single product. Combination reaction is also known as a synthesis reaction. Generally it is the exact opposite of chemical synthesis.
Chemical reactions Summarize the characteristics of synthesis decompositionand exchange reactions. Synthesis Reaction Examples In the simplest synthesis reactions two elements combine to form a binary compound a compound made of two elements. 2Na Cl 2 2NaCl.
For example under the influence of an electric field water breaks. In a Decomposition Reaction a complex substance breaks down into two or more simpler substances. Example of combination reaction.
This is a decomposition reaction. In a Synthesis reaction two or more simple substances combine to form a new more complex substance. An example is the burning of charcoal carbon in excess oxygen to make carbon dioxide.
The general form for a decomposition reaction is. Distinguish between chemical reactions that release energy and those that take in energy. In a decomposition reaction bonds are broken and a larger molecule is changed to two or more smaller ones.
In fact you may recognize this as a double-replacement reaction. The opposite of this type of reaction is a synthesis in which simpler reactants combine to form a more complex product. However an endothermic outcome is also possible.
A synthesis reaction is a reaction in which two or more reactants combine to form a more complex substance. Decomposition reactions require an input of some form of energy. The synthesis means combination of compounds and as the name says for these kind of reactions two or more substances combine to form you compound.
Correct Answer s. Synthesis reactions require energy for the formation of bonds. Practically synthesis is opposite of decomposition.
A combustion reaction occurs when a substance reacts quickly with oxygen. Decomposition reactions are chemical reactions where a reactant. The opposite of a synthesis reaction is a decomposition.
A decomposition reaction is the opposite of a synthesis reaction. The opposite of the synthesis reaction is called as the decomposition reaction. These substances can be compounds or elements.
Two different substances react to make two new substances. Some decomposition reactions release energy. Chemical decomposition is sometimes also referred to as chemical breakdown.
An example of a photo decomposition reaction is the decomposition into dioxygen and an oxygen radical as represented by the chemical equation provided below. In decomposition reactions a single complex reactant breaks down into simpler products like elements elements and compounds or just compounds. The newly formed product is always a compound while the reactants can be elements or compounds.
A photodecomposition reaction is a type of decomposition reaction in which the reactant is broken down to its constituents by absorbing energy from photons. Synthesis reactions are reactions that occur when two different atoms or molecules interact to form a different molecule or compound. A reaction in which a single compound breaks into two or more simpler compounds is known as a decomposition reaction.
In a decomposition reaction one reactant breaks down into two or more products. Most of the time when a synthesis reaction occurs energy is released and the reaction is exothermic. O3 hν O2 O.
Combustion is commonly called burning. The separation of a substance or material into two or more substances or materials that might differ from each other and from the original or unique substance are called as the. Find step-by-step Anatomy and physiology solutions and your answer to the following textbook question.
The breakdown of hydrogen peroxide to water and oxygen and the breakdown of water to hydrogen. These two reactions are opposite to each other. This does not fit the definition of either a composition reaction or a decomposition reaction so it is neither.
Decomposition reactions are also known as analysis reactions or chemical breakdowns. Explain how reversible reactions produce chemical equilibrium. Synthesis reactions are chemical reactions where two elements combine to make a product.
In the process of the decomposition reaction many complex compounds break down into multiple simpler compounds.

5 1 Synthesis And Decomposition Reactions Learning Goals Learn How To Identify A Chemical Change Learn What Is A Synthesis Reaction And How To Create Ppt Download

Expert Answer Differentiate Between Combination Reaction And Decomposition Reaction At Least 3 Brainly In

Decomposition Reaction Definition Examples Applications
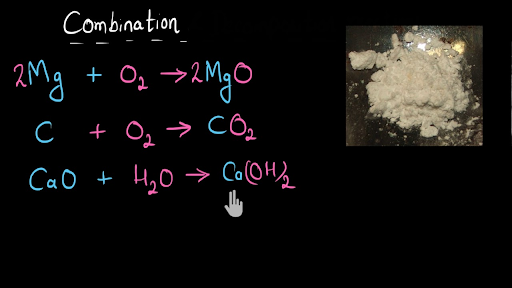
Combination And Decomposition Reaction Video Khan Academy

Chemical Reactions Combination Decomposition Combustion Single Double Displacement Chemistry Youtube

9i Differentiate Between Combination And Decomposition Reaction Give Example Ii Differentiate Brainly In
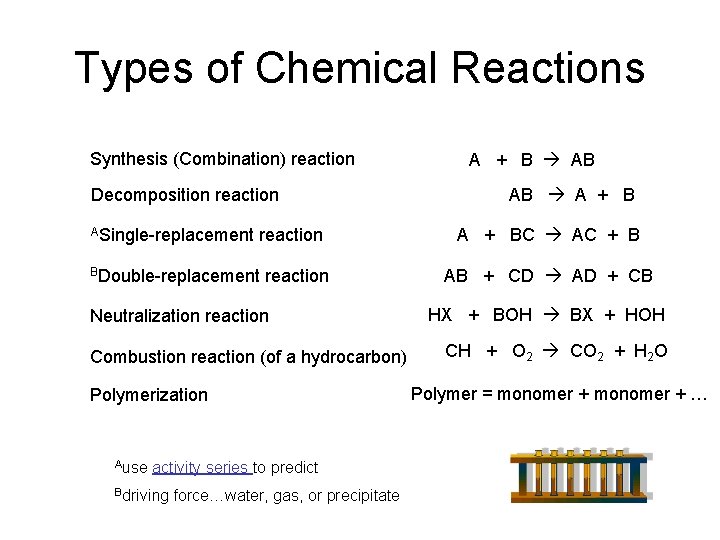
Types Of Chemical Reactions Synthesis Combination Reaction A

5 1 Synthesis And Decomposition Reactions Learning Goals Learn How To Identify A Chemical Change Learn What Is A Synthesis Reaction And How To Create Ppt Download

Synthesis Reactions Definition Examples Expii

Decomposition Reaction Definition Examples Applications

Difference Between Combination And Decomposition Reaction Compare The Difference Between Similar Terms
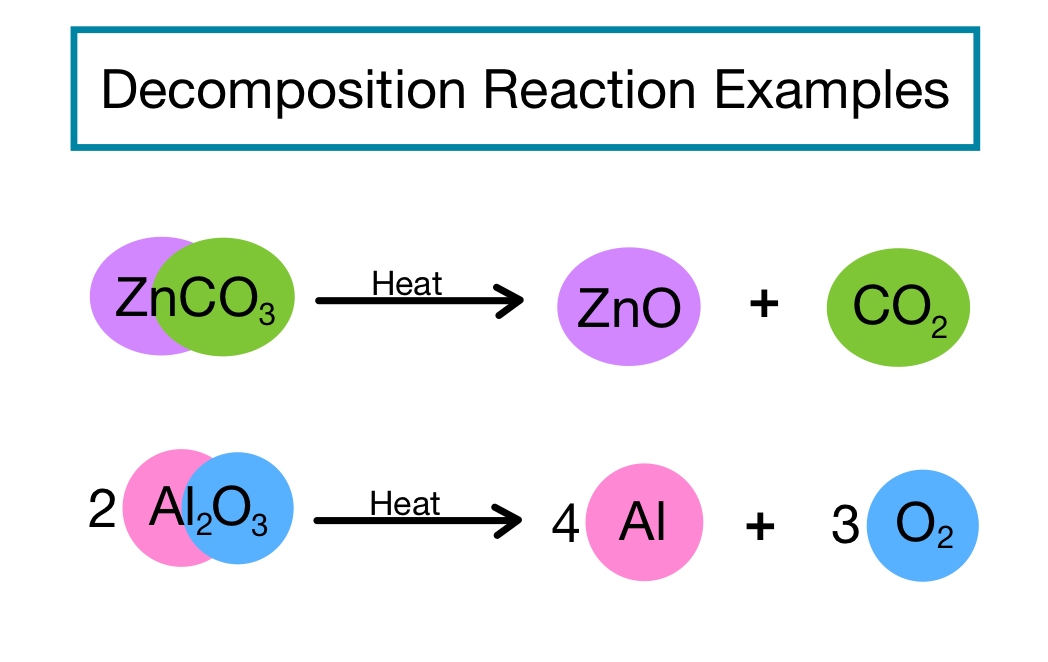
Decomposition Reactions Definition Examples Expii

Synthesis Combination Reaction Definition Examples And Applications

Decomposition Reaction Stock Illustrations 48 Decomposition Reaction Stock Illustrations Vectors Clipart Dreamstime
What Is An Example Of A Synthesis Reaction Quora

Types Of Chemical Reactions Synthesis Combination Reaction Decomposition Reaction A Single Replacement Reaction B Double Replacement Reaction Neutralization Ppt Download

Types Of Chemical Reactions Detailed Explanation With Example Videos
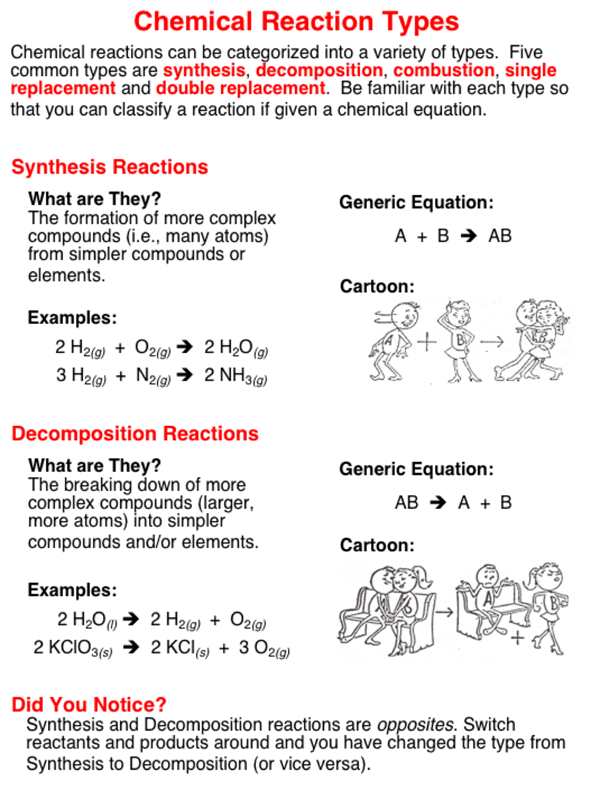

Comments
Post a Comment